Go Medical
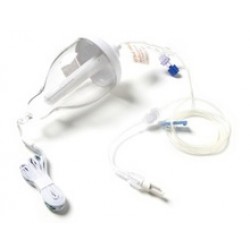
Nasal Spray PCA
The Go Medical Nasal Spray (PCINA) device is a hand activated nasal spray for the administration of drugs to the nasal mucosa. The device comes in two main variants, a Nasal Spray – No lockout (NS-NL) (Not for use with Opiate based analgesia) and a Nasal Spray – Lockout (NS-L) (for use with drugs with a risk of overdose e.g. opiate based analgesia). The lockout variants come in 2min, 3min and 4 min lockouts.The NS-NL variant delivers a nominal quantity of fluid in an atomized spray when administered by pressing the spray pump without any lockout. The device is not intended to be used for drugs that have a risk of overdose e.g. opioid based analgesics.The NS-L variants are intended for patient controlled analgesia (PCA) to administer suitable drugs or solutions via a spray to the nasal mucosa. The device delivers a controlled quantity of fluid in an atomized spray when administered by pressing the spray pump. The device is supplied non sterile and is intended for single patient use. The device is supplied empty and is intended to be filled prior to use.The NS-L variants are manufactured to lockout for a predetermined time between each full dose and are intended to be used to deliver a controlled metered dose over time of a lipophilic drug (e.g. fentanyl, sufentanyl) to the nasopharynx. If the user attempts to administer an additional dose before the lockout has occurred, the user will only be able to administer a partial dose. The lockout is controlled by Flow Control Tubing (tubing with a narrow bore that restricts flow rate) which limits the rate of flow to the pump chamber. The device is calibrated to deliver a full dose in a specified time interval. The device is intended to be filled before use by a pharmacist or health practitioner and used as prescribed...
O’Neil Balloonfusor
The O’Neil Balloonfusor is a non-electronic elastomeric infusion pump that is suitable for a broad range of applications. It can be used to provide intermittent or constant infusions by the Intravenous (IV) or Subcutaneous (SC) route, at a fixed flow rate set by the manufacturer. It is a light weight and portable allowing its use in a variety of hospital, healthcare and outpatient settings, including being worn by the patient during ambulation. The device is suitable for the administration of various drugs e.g. antibodies, anticoagulants, analgesics, parenteral anaesthetics, cytotoxics, anti-emetics, muscle relaxants and others such as Ranitidine, Phenytoin, Digoxin, Desferrioxamine. The Balloonfusor is supplied sterile and is intended for single patient use...
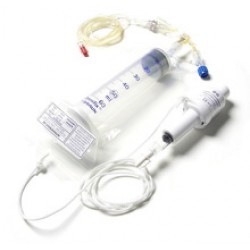
PCEA
The Patient Controlled Epidural Analgesic (PCEA) Device is a non-electronic, patient activated disposable pump designed to deliver pain management on demand and offers safety and ease of use as an advantage over electronic pumps.The PCEA is designed to make 4mL analgesic boluses available to the patient every 15 minutes, hence allowing the patient to deliver a maximum dose of 16mL per hour into their epidural space via an epidural catheter. The PCEA is ideal for labour wards, and post-operative gynaecological and orthopaedic patients. The device is lightweight and may be worn around the neck hence providing excellent mobility. Given that it is non-electronic, the device eliminates the need for pump programming. Human error is reduced and staff training is made easier. The affordability of this PCEA makes this form of pain management available to all patients...
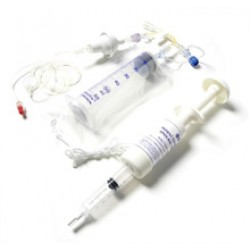
PCIA
The PCIA is a non-electronic, patient activated disposable pump designed to deliver intravenous analgesia on demand and offers safety, ease of use, portability and as distinct advantages when compared with electronically controlled delivery devices.The Patient Controlled Intravenous Analgesic (PCIA) Device is designed to make 0.5mL analgesic boluses available to the patient every 5 minutes, hence allowing the patient to inject a maximum dose of 6mL per hour via an intravenous catheter. The PCIA is ideal for patients who require post-operative pain relief and are able to operate the device unattended. The device is lightweight and may be worn around the neck hence providing excellent mobility. Given that it is non-electronic, the device eliminates the need for pump programming. Human error is reduced and staff training is made easier. The affordability of this PCIA makes this form of pain management available to all patients. ..
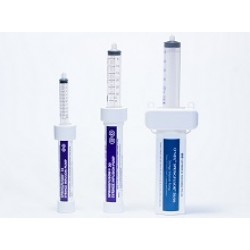
Springfusor® and FCT Range
The Go Medical Springfusor® Syringe Infusion Pump and Flow Control Tubing (FCT) is intended to provide constant Intravenous (IV) or Subcutaneous (SC) infusions in either a 10ml, 30ml or 50ml configuration at a variety of pre-set flow rates to cater for all needs.The Springfusor® syringe infusion pump is a low cost, non-sterile, reusable pump that requires no programming or external power source. It is simple to use and setup and requires only minimal training to load and operate (see video). The Springfusor® system is lightweight and portable, and can be worn around the patient’s neck or strapped to their arm to improve mobility during treatment.The Springfusor® syringe infusion pump is designed to be used with Go Medical FCT and accompanying syringe (sold separately) which can be easily connected to a patient’s cannula/port via standard luer lock fittings. The FCT and accompanying syringe are supplied sterile for single patient use and are available in a variety of flow rates and sizes depending on the variety of Springfusor® pump used. Flow rates range from 10ml/5min to 10ml/72hours for the Springfusor® 10-28, 30ml/5min to 30ml/96hours for the Springfusor® 30-AB, and 50ml/15min to 50ml/2h for the Springfusor® 50/60The infusion of fluids by the Springfusor® system is powered by the potential energy stored within a spring at the heart of the device. In the Springfusor® 10-28 & 30-AB, the spring is compressed by the action of loading the Springfusor® with the compatible syringe and FCT.The Springfusor® 50/60 incorporates two constant-force springs, which are extended when a syringe is loaded by hand into the cavity of the device.The Springfusor® is loaded by hand with the accompanying syringe attached to the required FCT. Fluids are delivered to the patient via the FCT attached to the patients cannula/port by the energy stored in the spring, which provides a constant force to the barrel of the loaded syringe. The flow rate is controlled by the FCT; wi..
Art. 1 de 5 - total 5 (1 Pág.)