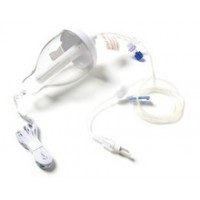
The Go Medical Nasal Spray (PCINA) device is a hand activated nasal spray for the administration of drugs to the nasal mucosa. The device comes in two main variants, a Nasal Spray – No lockout (NS-NL) (Not for use with Opiate based analgesia) and a Nasal Spray – Lockout (NS-L) (for use with drugs with a risk of overdose e.g. opiate based analgesia). The lockout variants come in 2min, 3min and 4 min lockouts.
The NS-NL variant delivers a nominal quantity of fluid in an atomized spray when administered by pressing the spray pump without any lockout. The device is not intended to be used for drugs that have a risk of overdose e.g. opioid based analgesics.
The NS-L variants are intended for patient controlled analgesia (PCA) to administer suitable drugs or solutions via a spray to the nasal mucosa. The device delivers a controlled quantity of fluid in an atomized spray when administered by pressing the spray pump. The device is supplied non sterile and is intended for single patient use. The device is supplied empty and is intended to be filled prior to use.
The NS-L variants are manufactured to lockout for a predetermined time between each full dose and are intended to be used to deliver a controlled metered dose over time of a lipophilic drug (e.g. fentanyl, sufentanyl) to the nasopharynx. If the user attempts to administer an additional dose before the lockout has occurred, the user will only be able to administer a partial dose. The lockout is controlled by Flow Control Tubing (tubing with a narrow bore that restricts flow rate) which limits the rate of flow to the pump chamber. The device is calibrated to deliver a full dose in a specified time interval. The device is intended to be filled before use by a pharmacist or health practitioner and used as prescribed.
| Caracteristicas | |
| Caracteristicas | Product History Initial development work for the nasal spray was started in 1997. The product has been manufactured since 1997 and sold in Australia, Europe and the US. In the US, the Nasal Spray is labeled as a Nasal Inhaler as it is not classified as a medical device. Indications For the administration of suitable lipophilic drugs with a viscosity of around 1.0 mPa.s, via the nasal mucosa in the form of an atomized spray. Contraindications The No-Lockout variant is not intended to be used with drugs with a risk of overdose, such as opioid based analgesics. Device Variants Nasal Spray PCA – No Lockout |